
Lactic Acid Fermentation in Sourdough
A few years ago, I was asked to explain lactic acid fermentation in sourdough, and the difference between homo- and heterofermentation. Not an easy task, partly because I wasn't satisfied that I knew enough, or that I could reconcile what I was reading in bread-baking books with what I had learned in school. To sort it out, I had to dig deeper into the scientific literature. Answers are there in bits and pieces, although not in a context that is easy to make sense of. As I plugged away at deciphering current microbiology textbooks and scientific research, I started to see things in a new light. And so now, I want to share what I've learned with those who wish to know more.
First, I'd like to introduce the concept of a metabolic pathway. On paper, a metabolic pathway can be illustrated in a flow diagram that represents a sequence of enzyme-controlled chemical transformations. While the pathways in this discussion start with sugar and finish as various end-products, there are several intermediate compounds formed along the way as one thing is converted to the next. The names may be intimidating at first glance, but don't let them scare you. Knowing their chemical reactions and what all the compounds are is not as important here as understanding their overall purpose, which is to produce energy for the organism. Like all living things, microbes need energy to perform the tasks that enable them to live, grow and multiply.
Some pathways generate more energy than others. Through respiration, glucose and oxygen are turned into carbon dioxide and water via the Krebs cycle, also known as the tricarboxylic acid or TCA cycle. You may have seen it before if you've studied biology, because it's the same pathway we humans use. It is aerobic, meaning that oxygen (O2) is involved, and it generates far more energy than any fermentation pathway. Whenever oxygen is available, respiration is favored by facultative anaerobes like yeasts, because they will always take the path that generates the most energy under the prevailing conditions. For the most part though, bread dough is anaerobic (without oxygen), and fermentation is an alternative pathway that doesn't require oxygen. When yeasts ferment sugars, they produce alcohol (ethanol) in addition to carbon dioxide. Fermentation produces much less energy than respiration, but it allows microorganisms to carry on when no oxygen is available, or they lack the ability to respire as is the case with lactobacilli.
Bacterial fermentation is more varied than fermentation by yeast. Bacteria produce organic acids that contribute, for good and bad, to the quality of bread. Controlling acid balance and degree of sourness is something that artisan bakers strive to do, so it may be useful to understand where the acids come from and how their production can be influenced by things that are within the baker's control. In yeasted breads, acids come in small doses from naturally occurring bacteria present in flour and commercial yeast. (Fresh yeast generally has more bacterial inhabitants than dried, and whole grain flours more than refined.) In sourdough breads, acid-producing bacteria are supplied in much greater numbers from starter. There are many different species and strains of bacteria found in various types of starters, and because they produce lactic acid while fermenting sugar, they fall under the heading of Lactic Acid Bacteria (LAB).
Lactic acid bacteria common to sourdoughs include members of Leuconostoc, Pediococcus, Weissella and other genera. But by far, the most prevalent species belong to the very large and diverse genus, Lactobacillus. Based upon how they ferment sugars, lactic acid bacteria can be sorted into three categories. Please bear with me now, because while these terms may look impossibly long and technical, they are actually self-descriptive. Take homofermentative LAB for example. Homo-, meaning "all the same," refers to the end product of fermentation (by lactic acid bacteria), which is only, or "all" lactic acid. Heterofermentative then, means "different" or mixed end products. As lactic acid bacteria, heterofermentative LAB produce lactic acid, but they also produce carbon dioxide gas, alcohol or acetic acid as well.
SUGAR STRUCTURE
As carbo-hydrates, sugars are made up of carbon (C) and water, which is composed of hydrogen (H) and oxygen (O). The hydrogen and oxygen atoms are arranged in various configurations around a chain of carbon atoms which form the structural backbone of the molecule. The carbon chain may be various lengths, but sugars common in bread fermentations are of the 5- and 6-carbon types, referred to generically as pentoses and hexoses, respectively. Glucose and fructose are examples of hexoses. Pentoses are sugars such as arabinose and xylose.
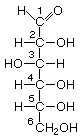


Glucose Fructose Arabinose XylosePentoses and hexoses can exist in the chain form, or in a ring structure which forms when dissolved in water. Single sugars, or monosaccharides, are often linked together into larger carbohydrates of two or more units. Disaccharides, containing two sugars, are important in bread fermentations. Maltose, which is made up of two glucose molecules, is the free-form sugar most abundant in dough. Sucrose, another disaccharide consists of one glucose and one fructose.
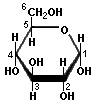
Glucose Maltose

Fructose Sucrose
-Sugars illustrated by Antonio Zamora. For a more complete explanation, with diagrams of starches and pentosans, please see his lesson, "Carbohydrates - Chemical Structure" at: http://www.scientificpsychic.com/fitness/carbohydrates.html
Sugars that can be fermented, and their end-products are variable from one species of LAB to the next. But the key lies in the structure of the sugar---particularly, the number of carbon atoms in the backbone of the molecule. Homofermentative LAB can only ferment 6-carbon sugars. In the homofermentative pathway, a hexose is processed and split into two identical 3-carbon pieces, which are passed down through the reaction sequence and transformed into lactic acid molecules. In contrast, heterolactic fermentation is based on 5-carbon sugars. Pentoses may be used directly, although more often, a hexose is cut down by removing one of its carbons. The extra carbon is cast off in the form of carbon dioxide gas, and the remaining 5-carbon molecule is split unequally into 3- and 2-carbon units. The 3-carbon piece is turned into lactic acid, while the 2-carbon piece will become either ethanol or acetic acid. Up to this point, heterolactic fermentation doesn't produce as much energy as homolactic, but it does give an advantage over homofermentative LAB, which cannot utilize pentose sugars.
Additional energy can be produced by turning the 2-carbon piece into acetic acid, but it requires the assistance of another substance. The term for this is co-metabolism, meaning that two substrates are used simultaneously---a hexose for its carbon backbone, and a co-substrate to facilitate the formation of acetic acid and generation of additional energy. The co-substrate can be one of a number of things including oxygen, citrate, malate, short chain aldehydes, oxidized glutathione, fructose and 5-carbon sugars. In the absence of co-substrates, the 2-carbon piece is turned to ethanol instead. Alternatively, when pentose sugars are fermented (used as the carbon source), acetic acid may be produced without the help of co-substrates.
Some lactobacilli can use oxygen as a co-substrate. Some cannot, and are inhibited by aerobic conditions. In any case, there is a small amount of oxygen in dough only at the beginning of fermentation, and generally not enough to affect acetic acid production to any extent. Likewise, citrate and malate aren't naturally present in significant amounts, and pentose utilization varies by species and strain as well as availability. While all these things may be used to the extent that they are present, it turns out that fructose is generally the one most available in bread dough.
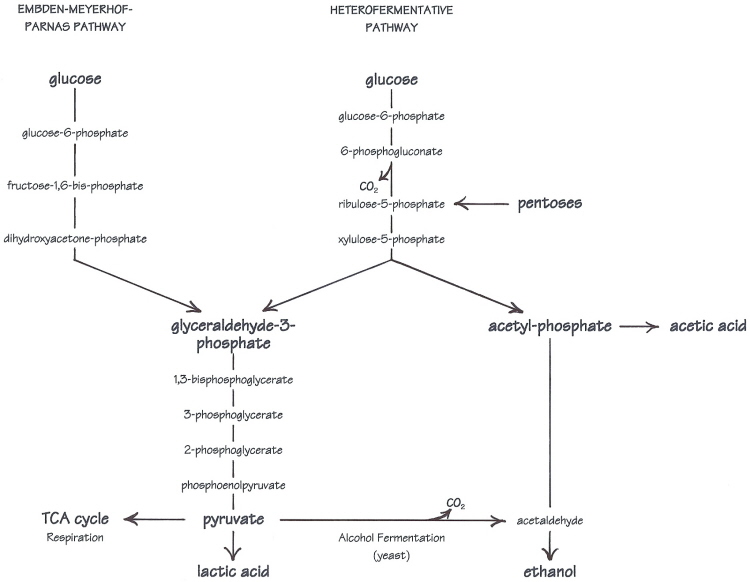
All of the pathways in this discussion are glycolytic pathways. Glycolysis is the conversion of glucose to pyruvate, which is the springboard to both respiration and alcohol fermentation in yeast, to lactic acid fermentation in LAB, and to many biosynthetic pathways (manufacture of compounds used in other life processes). Oxygen is not required, so glycolysis is especially important to microorganisms that ferment sugars, like the yeast and bacteria which grow in the anaerobic environment of sourdough.
Homofermentative lactobacilli share the same glycolytic pathway with yeasts---the Embden-Meyerhof-Parnas, or EMP pathway. But in contrast to alcohol fermentation, pyruvate is reduced to lactic acid. In either of the two pathways here, the sugars are split into smaller molecules---two identical 3-carbon units (glyceraldehyde-3-phosphate) in the EMP pathway, or a 3- and a 2-carbon unit in the heterofermentative pathway. The 3-carbon pieces all follow the same path to become pyruvate and then lactic acid, while the 2-carbon acetyl-phosphate on the other side of the heterofermentative pathway can become either ethanol or acetic acid.
Glucose is not the only sugar that can be utilized. With appropriate enzyme systems, other sugars can be converted into glucose or one of the intermediates in the pathway such as glucose-6-phosphate (or in the case of pentose sugars, ribulose-5-phosphate). The ability to use other sugars varies by species and strain. Most sourdough lactic acid bacteria ferment glucose preferentially, but Lactobacillus sanfranciscensis separates maltose into a glucose-1-phosphate and a glucose. The glucose-1-phosphate portion is converted to glucose-6-phosphate to enter the heterofermentative pathway, and glucose is excreted from the cell.
In addition to obligately hetero- and homofermentive, there is a third type of lactobacilli characterized as facultatively heterofermentive. These are lactobacilli that are not restricted to one pathway or the other, but can use both. Facultative heterofermenters switch back and forth between the homo- and heterofermentative pathways depending upon which sugars are available. In general, they ferment hexoses via the homofermentive route, and pentoses heterofermentively. Most will use the hexose sugars first, although some strains ferment pentoses preferentially. Many co-metabolize fructose with maltose through the heterofermentative pathway, but use the homofermentative pathway when only maltose is available.
To put all this technical information to practical use, we need to consider factors that influence LAB activity and pathway selection. The end products are determined by the species and available sugars, which for lean doughs, depend upon the flour and the activity of enzymes. Whole grain and high extraction flours can affect acidification in two ways. First, the higher mineral (ash) content serves as a natural buffer system, which allows bacteria to produce more acid before the pH drops low enough to slow their growth. And second, grains supply pentose sugars in the form of pentosans. Although rye flours are best-known for these, pentosans are also present in wheat and other grains. (But, because they occur in the outer layers of the kernel, they are largely removed along with enzymes and many other substances in the milling of refined flours.) Cereal enzymes act on pentosans to some degree, freeing pentose sugars like xylose and arabinose that heterofermenters may be able to use according to species and strain. Pentoses will increase acetic acid production if they can be fermented or co-metabolized, either one.
Acidification is also influenced by hydration and temperature. Contrary to popular belief, all three groups of sourdough lactobacilli prefer wetter doughs a bit on the warm side, many growing fastest at about 90ºF or a little higher. For the homofermentive species producing only lactic acid, increasing activity by raising the hydration and/or temperature will increase acid production. Decreasing activity by reducing hydration or by retarding will slow production. There is a direct relationship between activity and lactic acid. During heterofermentation, for each molecule of glucose consumed, one lactic acid is produced, along with one carbon dioxide (if a hexose is fermented), and either one ethanol or one acetic acid. But under wetter, warmer conditions, where sugars are metabolized more rapidly, the tendency is toward lactic acid and alcohol production in obligate heterofermenters, and all lactic acid (homofermentation) in the facultative heterofermenters. Lactic acid production is directly related to activity during heterofermentation just as in homofermentation, even if only half the rate.
At lower hydrations and temperatures (lower activity), more acetic acid is produced, but not because of temperature per se. Acetic acid production is influenced indirectly by temperature, in that it affects the kinds of sugars available. The fructose that drives acetic acid production, is liberated from fructose-containing substances in flour, largely through the enzyme activity of yeast. And, because lower temperatures are more suited to yeast growth than higher, more fructose is made available to the bacteria at lower temperatures. At the same time, the bacteria are growing and using maltose more slowly, so the demand for co-substrates goes down as the fructose supply goes up. The ratio of acetic acid to ethanol and lactic acid goes up, because a higher percentage of the maltose is being co-metabolized with fructose. Reducing hydration has a similar effect of slowing the bacteria more than yeast, which I believe is the real basis for increased acetic acid production in lean breads made with refined flours.
Contrary to myth, the species that grow in sourdough starters are not tied to geographic location, but rather to the traditional practices in the different regions. Several organisms go into the mix, but the environment created inside the starter from the combination of flour, temperature and maintenance routines is what determines which ones will thrive. In type I, or traditional sourdoughs (i.e., those maintained by continuous refreshment at room temperature), the obligately heterofermentive Lactobacillus sanfranciscensis is the species most frequently and consistently found---not just in San Francisco where it was first discovered, but all around the world. And so it deserves special attention.
Lactobacillus sanfranciscensis is fairly unique among the obligately heterofermentive lactobacilli, in that it ferments no pentose sugars. And unusual among lactic acid bacteria in general, because it prefers maltose over glucose. But it will co-metabolize fructose with maltose to produce acetic acid. L. sanfranciscensis converts maltose into one glucose-6-phosphate which enters the heterofermentative pathway, and a glucose which is excreted back into its surroundings. This is a good arrangement for common sourdough yeasts, since maltose is the most abundant sugar in wheat doughs, and some lack the ability to break it down for themselves. Yeasts and other bacteria that can ferment maltose, generally prefer glucose. And so by providing glucose to competing organisms, L. sanfranciscensis actually helps to conserve the maltose for itself---just one of the ways in which it gets along well with other sourdough microorganisms, and perhaps one of the reasons it is found so often.
Alternate pathways are a recurring theme in the microbial world, because microorganisms have less ability to control their environment or to leave when conditions become difficult. They sometimes have to switch gears to survive. In that effort, lactic acid bacteria will utilize whichever fermentation pathway that generates the most energy within their capabilities and resources. In order of preference, the hierarchy seems to be heterofermentation with co-substrates (forming lactic acid and acetic acid), followed by homofermentation (all lactic acid) and heterofermentation without co-substrates (lactic acid and ethanol).
While traditional sourdough starters usually support one or more strains of Lactobacillus sanfranciscensis, it is often found in combination with the facultatively heterofermentive Lactobacillus plantarum, many strains of which can either ferment or co-metabolize at least one pentose sugar. Various other obligate and facultatively heterolactic acid bacteria are also common (obligately homofermentive LAB are only transient in the startup process and do not persist in established type I starters). Sourdough starters are sensitive ecosystems with complex associations of lactic acid bacteria, and combinations can be highly variable from one starter to the next. Lactic acid fermentation is as complex and varied as the organisms involved, and so sourdough processes may need to be optimized on a starter by starter basis.
- Debra Wink
Bibliography
Arendt, Elke K., Liam A.M. Ryan, and Fabio Dal Bello. 2007. Impact of sourdough on the texture of bread. Food Microbiology 24: 165-174.
De Vuyst, Luc and Marc Vancanneyt. 2007. Biodiversity and identification of sourdough lactic acid bacteria. Food Microbiology 24:120-127.
Doyle, Michael P., Larry R. Beuchat, and Thomas J. Montville. 2001. Microbial physiology and metabolism, p. 19-22; Lactose metabolism, p. 653-655. Food Microbiology Fundamentals and Frontiers, 2nd ed. American Society for Microbiology Press, Washington, DC.
Gänzle, Michael G., Michaela Ehmann, and Walter P. Hammes. 1998. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Applied and Environmental Microbiology 64:2616-2623.
Gänzle, Michael G., Nicoline Vermeulen, Rudi F. Vogel. 2007. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiology 24:128-138.
Gobbetti, M., P. Lavermicocca, F. Minervini, M. De Angelis, and A. Corsetti. 2000. Arabinose fermentation by Lactobacillus plantarum in sourdough with added pentosans and "alpha-L-arabinofuranosidase: a tool to increase the production of acetic acid. Journal of Applied Microbiology 88:317-324.
Holt, John G., Noel R. Krieg, Peter H. A. Sneath, James T. Staley, and Stanley T. Williams. 2000. Regular, nonsporing gram-positive rods, p. 566. Bergey's Manual of Determinative Bacteriology, 9th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
Katina, Kati. 2005. Sourdough: a tool for the improved flavour, texture and shelf-life of wheat bread. VTT Technical Research Centre of Finland.
Ng, Henry. 1972. Factors affecting organic acid production by sourdough (San Francisco) bacteria. Applied Microbiology 23:1153-1159.
Paramithiotis, Spiros, Aggeliki Sofou, Effie Tsakalidou, and George Kalantzopoulos. 2007. Flour carbohydrate catabolism and metabolite production by sourdough lactic acid bacteria. World J Microbiol Biotechnol 23:1417-1423.
Wing, Daniel, and Alan Scott. 1999. Baker's Resource: Sourdough Microbiology, p. 230. The Bread Builders. Chelsea Green Publishing Company, White River Junction, VT.
This article was first published in Bread Lines, a publication of The Bread Bakers Guild of America. Vol. 15, Issue 4, Dec. 2007.
Revised: November 4, 2009





Debra,
I won't say that I understood everything that you wrote, but thank you very much for the parts that I did understand.
From an amateur baker's perspective, what ought I be doing to maximize flavor and aroma in my finished breads? Am I trying to optimize for lactic, or acetic, acid production? Or do I need a regime (Detmolder comes to mind) that favors each at different points during fermentation? And what tactics can I employ to make sure that the yeast remain healthy and active while the bacteria are making acids?
I guess I'm trying to make the connections between the microbiological and biochemical information that you have provided and the strategies, tactics and processes that I'm able to employ in my kitchen.
Thanks again for assembling and organizing all of this information in a concise form.
Paul
Thank you Paul, and everyone else too! As a fellow seeker of answers, let me first say that as fascinating and wonderful as the world of science is, the more I learn, the more I appreciate just how complicated and magical a good loaf of bread really is. The process will never be distilled into a simple equation, because it's just never that simple with living things. Bread is truly an art. You can paint by numbers following a recipe, or you can follow your own inspiration with all the colors and tools in your own toolbox.
The question, "What is artisan bread?" generally leads to "What makes someone an artisan?" I'm not going to try and define that for everyone else, but I think that experience, knowlege and wisdom/instinct for one's craft certainly are aspects. A true artisan understands his medium, its strengths and limitations, and how to manipulate it. This paper was meant to add a dimension to the knowledge part, and hopefully dispell a few common myths---like heterofermenters (acetic acid producers) grow faster at lower temperatures. They do not. And understanding that may help you decide how and when to use temperature (or hydration/flours/ingredients) as a tool.
In the literature, 80% lactic to 20% acetic acid, give or take a little, is generally considered the most desireable ratio for flavor. However, since we have no way of measuring that at home, the numbers are really of no use. Like with wine, to each his own. Experiment with different methods, observe the effects, and let your palate be the deciding factor. Many of you are more accomplished bread bakers than me, so I generally don't try to advise people how to make good bread :-) There are more things to consider than making more or less acids for flavor, because they do have an effect on dough rheology and rise as well.
So, I guess what I'm really telling you is that I give you this information to add to your toolbox. But I'm leaving it up to you to decide when and how you'll use it.
...I'm sure Peter would say Amen to that. ;-)
http://peterreinhart.typepad.com/
God's blessings,
Mark
...You're the best. ;-)
I knew you would like this :-)
Hi, Debra.
Now, why couldn't you have been my microbiology professor!
This is a wonderfully lucid summary of a very complex subject.
I do have a suggestion/request on behalf of the SD bakers without your scientific background: Could you summarize the specific implications of the metabolic knowns for bread baking. How can we manipulate time, temperature, hydration and ingredients to achieve more or less production of acetic and lactic acid?
Thanks for sharing your expertise with us.
David
Thanks David!
Well, everything is relative, but I guess in general
1) more time generally means more acid. But see note*
2) higher temperatures mean acids accumulate faster, with a higher ratio of lactic to acetic. Lower temperatures produce acids slower, but the percentage of acetic increases. See note*
* NOTE: The limiting factor for lactic acid is the buffering capacity (ash content) of the dough, while the limiting factor for acetic is availability of co-substrates (electron acceptors for you chemistry types).
3) Lactobacilli prefer wetter doughs. Yeast don't seem to mind low hydration (or salt or low pH) as much as bacteria.
4) Flour plays a big part---ash content, enzyme activity, etc. This is where the sugars and co-substrates come from for lean doughs, so the type matters. Whole grains or higher ash generally result in more acetic acid and more total acid.
5) Your results may vary, depending on what species of LAB you're growing in your starter.
6) It's never that simple with living things :-)
See, this is why I said we need you.
Of course, I'll have to re-read that several times, and I think I'll have a bunch of questions. But, that was more solid scientific information about sourdough than the sum total of everything I had read before.
I'm so glad you jumped in here.
Dave
That was a great read. I have been looking for that kind of information for a while. Thanks.
Thanks for such an easily digested (ahem) presentation of the LAB world. I have often referred to the Ganzle et al modeling paper that you cite, and your explanation answers a lot of questions. I still have some, though. In his paper, as I understand it, Ganzle uses growth rate as the parameter of interest, and models (plots) it against several variables such as temperature, salt, pH, etc. Is growth rate a good substitute for the rate of CO2 and acid production, which I think is what we are interested in as bakers? I understand that we need to grow a large healthy population of microbes in our starter and dough, but is there some condition that might not favor reproduction, but CO2 generation for instance instead? I'm thinking of the baking phase, but maybe there's a less catastrophic environment, from the microbes' point of view, that would be useful.
Another question relates to the populations of microbes in starters. If I make a fresh starter every week from a mother starter that I keep refrigerated, unrefreshed for months at a time, should I expect that the weekly starter will have the same population (ratiometrically) every time? (I promise to keep everything the same each time: temp, flour, water, hydration.) Refreshing the mother starter is just a matter of taking an active weekly starter, reducing the hydration by adding flour, and refrigerating it. Or do I have to keep the starter on the countertop and refresh it daily, similar to having another pet to watch over (or owning a commercial bakery that never closes for more than a day).
Finally, I have read that the lb sf has never been isolated in the 'wild', only in bakery starters. Is this true, and if so, is it unlikely that the LAB in our starters that we made from scratch is actually lb sanfranciscensis?
Thanks again for the great article. I'm sure it's being marked as favorite right now by many readers. Here's hoping your patience holds out with all our questions.
Stewart
Stewart, your last question is really the essence of the issue for us home bakers. I don't keep my starter at room temperature for long periods because of the daily attention it would require. It is much easier to feed and refrigerate the mother and use it as needed. I get decent results but I have had the feeling that I may be sacrificing some flavor component by using such a cool environment.
Probably a better approach would be to bring the storage starter to room temp, elaborate an amount for the days bake and feed the storage amount, planning on leaving it out for another feeding the next day. Hopefully the balance of the biology would remain stable once it was placed back in the cooler for the next use.
There is a flavor component in the original SF sourdough that is unique. I haven't tasted it for a while but the Wharf bread I ate on Fisherman's Wharf many years ago was unbelievably delicious. It may be that those flavors are simply a result of adjusting the feeding procedure to encourage the proper balance of the right organisms that are needed to produce flavor components and, raise the dough vigorously.
As you say, it may be we just need to start treating our starter like a house pet. If I start giving table scraps to the starter pet, my wife is gonna kill me:>)
Eric
Because my job usually requires extensive travel, I normally refrigerate my starter.
However, for the past several months I have been working from home and have been able to keep my starter as "free range" and feed it daily. I know it should be twice daily, but it really doesn't look "hungry" after 12 hours. My house is kept pretty cool.
Yes, I "waste" starter/flour feeding it (Look, it's a fairly inexpensive hobby...) I've gotten the routine down and it takes very little effort per day.
What I am observing is not so much a difference in taste or pH, but just a more reliable and lively rise and better dough qualities. It's just a happier baking experience. Not objective, I know, but there you are.
Given the choice, I would never go back to the refrigeration method. Feed the cat - then feed the levain. Ten minutes well spent...
For what it's worth...
Pat
Stewart, I don't have enough time right now to do justice to your questions, but I'll try and get back to you soon. The short answer is that CO2 generation and growth rate do corollate somewhat, but they're not maximized at exactly the same point. I did say something about optimal temp in the sourdough 101 thread that is related to this though. One thing to keep in mind, is that what you want depends on the situation. You need gas production to raise bread dough, but in maintaining a seed culture, population growth is probably more the goal. Depends on your purpose.
Ah, the mystery: Where does L. s. come from? Well, it comes from somewhere ;-)
to be continued.....
Thanks again for the article and also for your quick responses to our questions. I'll check the SD101 thread about optimal temps, and I'll be interested to follow your future postings.
Stewart
Stewart, addressing your first paragraph may be easiest if we do a little microbiology 101. Think of energy as currency in an account where deposits and withdrawals are being made all the time. Like you going to work at your job to generate the income you need to pay for your living expenses, savings and fun money, a cell goes to work metabolizing sugars to generate energy that the cell spends on homeostasis, growth and division. As with income, various things affect both the rate at which energy is produced and the rate at which it is spent. Things like temperature, food supply and metabolic pathway (aerobic or anaerobic) have a big effect on how much energy is generated, but things that effect energy consumption have a big impact too. Conditions (temp, hydration, salinity, pH, etc.) affect how much energy it takes to perform tasks and by what means. There is a hierarchy to how energy is spent. First is homeostasis, or the basic cellular processes to maintain life---survival. If there is energy left over, it goes into growth. And if enough energy, then division.
pH is as important to microbes as temp, water, food, etc. Because single-cell creatures are in direct contact with their environment, acid (H+) can cross the cell membrane into the cell. The inner working parts of the cell are made up partly of proteins, and when proteins meet with acids, they coagulate. To keep them from "cooking" and shutting down cellular machinery, the pH inside the cell must be regulated by pumping out excess H+ as necessary. Pumping H+ out of the cell requires energy, and the lower the pH drops, the more the cell must pump.
Water activity is another biggie. I'm not sure how to explain it except to say that all living systems require water to efficiently carry out life processes. Water activity is a measure of the availability of water, or hydration/dehydration. It's influenced not only by the amount of water in the surrounding environment, but also by solute concentration (namely sugar and/or salt), because solutes in the environment draw water out of the cells. Put another way, water moves toward the area of higher concentration of solutes. To counter dehydration, cells must pull compatible solutes into the cell so that water will move the other way. This is another active process requiring energy. And, the more energy that must be spent to hydrate the cell, the less energy it has to reproduce itself.
The amount of energy available for division depends not only on how much is created through carbohydrate metabolism, but also on how much must be spent on homeostasis. The farther from ideal the various conditions are---temp, hydration, pH, salinity, etc.---the less efficient a cell becomes. Stack up enough factors that are less than ideal for the cell, and it must spend more and more of its available energy on survival. So, CO2 is an indicator of carbohydrate metabolism and energy generation in yeast (lactic acid for LAB), but it's not necessarily an accurate indicator of population growth.
The second paragraph I'm not sure if I understand the question. You are keeping a mother starter unrefreshed for months at a time, and drawing on that to make your working starter for bake days? If it is unrefreshed, it is likely to go deeper and deeper into dormancy, and take longer and longer to wake up over time. I can't speak to the population numbers, bacause I've never heard of a study that's been done to measure that under the conditions you're describing. But I guess I can say this: A starter will likely remain stable (be that good or bad) as long as it is maintained it stably---do the same thing on the same schedule---once an equilibrium has been reached.
In the most current literature that I have read, Lactobacillus sanfranciscensis is considered to be the most common LAB found in type I starters---those maintained by continuous refreshment at room temperature (like they are in bakeries). I think it takes a couple weeks at room temp after getting a new starter up and running, before Lb. sanfran. gets going, so I'm doubtful that it would be in one that is refrigerated right away. There was a paper done about population dynamics in starters under different sets of conditions. Unfortunately it didn't include any starters kept below warm room temperature, so I can't say with any certainty that L.s. persists in refrigerated starters. But I imagine it would have a better chance in starters that are refreshed regularly and allowed to spend a refreshment cycle or two out of the refrigerator each time.
Hopefully I answered your question :-)
A while back bwraith and I were speculating on what's happening inside my levain (about which I have no complaints, but also which most "sourdough experts" tell me should be dead). He brought up the possibility that since I keep a relatively large amount of levain in fairly cool conditions (50-65F) and refresh it at a relatively low ratio (I do it by eye and it's probably 1:1:1) that I might be encouraging l. pontis which tends to appear in in commercial cultures maintained in this way. Do you have any insights on the differing strains of lactobacilli and why they appear?
We speculated that if I changed my feeding routine to something like 1:5:5, I would begin to see a change. However after several months of this new routine - nothing seemed to be different. No pH changes, no growth rate changes (that was really wierd, I thought), nothing. Here is an example where I drastically changed a feeding schedule, but the starter seems to be so stable (The original was started about 5 years ago) that nothing will move it. Do you have any insights there?
So many questions. Thanks for what you have contributed so far!
Pat
Pat, this is such a good question, because it points back to the common misconception that starters kept cool will get very sour. The belief is based on the notion that heterofermenters---the ones that produce acetic acid---like it cool and grow faster at lower temperatures. But as I said earlier, they do not. It's just the opposite in fact. And I think this is a really important thing to understand. Even though at cool temperatures, they produce more acetic acid in proportion to lactic and ethanol, they are producing the acids at a slower rate, and they are growing and multiplying slower as well.
I think that the commercial cultures you are referring to are the type II sourdoughs, maintained at a high temperature (>85F) with low refreshment rate and long ripening time. Yeast don't do well in that environment because of the temperature, nor does Lactobacillus sanfranciscensis because of the low pH. Cultures maintained this way get very sour and lose their yeast. They are used for flavoring but not for leavening. And L. pontis is common in this type of sourdough.
Even though you are using a relatively low refreshemnt rate, the low temperature creates the opposite effect on your starter. Lactobacilli slow way down; and yeast slow down too, but not as much. Because the lactobacilli slow down and don't divide as quickly, the population shrinks in relation to the yeast. And acids don't accumulate vary fast, because 1) there are less bacteria to make them, and 2) the bacteria that are there are producing the acids more slowly. At the same time the yeasts have less competition from bacteria for sugars, and less acetic acid to contend with, so they have a tendency to do fairly well. For this reason I think you'll find that starters maintained cool get less sour and rise quite well between refreshments. Has that been your experience?
This is what keeps desem mild. 100% whole wheat sourdoughs have a tendency to get very sour, because of the ash content and greater supply of co-substrates. But keeping it cool keeps the bacteria in check, and the yeast robust.
If this seems confusing in comparison to retarding dough to increase the sour, just think of it as a numbers game. In dough, you spend time building the bacterial populations in the warmth, building/ripening your starter, and bulk fermenting the dough. Then, you cool it down (and give it more time) in the retarding/proofing stage to draw more acetic acid out of those bacteria. Do you see how that is different from keeping the bacterial populations from building to begin with?
And I'll bet that the reason you didn't see much difference in growth rate is because you only added 2 generation times. Look at your refreshment rate as a dilution factor. 1:1:1 is a 3x dilution of your starter. That's less than two doublings or "generation times"---the time it takes the population to double. 1:5:5 is 11x, or less than 4 generations. In either case, there's a long lag time at the beginning before they start reproducing, and then they ramp up in speed with each generation, until food starts running out. You can think of it a little like flying. It takes an airplane some time and distance to get up to cruising altitude, but once there, you can cover more distance and make up for lost time :-)
Oh, and cool starters are more stable than warm. I'm just guessing here, but maybe that's because the pH doesn't fluctuate as much from refreshment to refreshment.
What you predicted is exactly what I experience. My levain is a bit on the mild side, but rises quite well.
What that means is - for me - who doesn't like the really sour bread (I hung out in the Bay area for quite a while and never did like the San Francisco sourdough. It's our differences that make the world go 'round) and does not want to use commercial yeast - I might as well keep on keeping on. I've got the levain that I want.
So, if I understand correctly, what you are saying is that I have the lactobacilli that I have. Refreshment rate will not change that - it will mostly change the vigor of the lactobacilli. Correct?
Thanks for all of your work on these and other qestions
Pat
Thanks Debra for the (again) in-depth reply. The feeding frenzy of having a microbiologist in our midst may die down, or then again, it may not. I hope you get something out of the bargain. We certainly appreciate your time and attention.
I think I'll continue to use the graphs in Ganzle's 'Modeling' paper as my guideline for my starters. (In case you want to address them by name, Izzy is Isola Ischia, Kamal is Camaldoli, and Riley is my new rye starter.) The second question is as you guessed, the stability of a refrigerated starter. My two Italian starters Izzy and Kamal have been maintained with this regimen for a year or so, with no apparent change in taste or behavior (very little sour flavor). Riley is brand new and already quite sour, so it will be interesting to see if it maintains its acidity over time. As for lb sf, it would be interesting to know if I have it in Riley, but it really doesn't make much difference, if it continues to make good bread.
I yield the balance of my time to the next TFLer.
Stewart
Debra,
Thanks for the great explanation. However, depending on the intended scope of your article, one is left with the impression that yeast activity in a sourdough starter is relatively unimportant. Is that correct?
cb
Thanks cb---I chose to focus on lactic acid fermentation because that is the area that is the most complicated and lacking in information. Yeast activity is very important, because it's yeast raising the dough for the most part, not bacteria. But information on alcohol fermentation is relatively easy to come by :-)
I missed this excellent post earlier. Great information! And a whole lot of work. Thanks, Debra! I'll be coming back to this post. Lots of info to mine here.
There is a lot to think about in your post. Thanks for it.
Well done, Debra, thank you for the time you put into this.
Perhaps sourdough fermentation deserves its own baker's software model! (err, perhaps the biotech industry has already done that I'm sure), but wouldn't it be nice for bakers to have a program where you could plug in flour type, temperature, hydration, time, etc., etc., etc., and get an output likely population of microbes with their corresponding range of acetic to lactic acid ratios that impart a corresponding range of tastes. (I know, taste is subjective, and a model takes the art out of baking, but I am a scientist by nature...).
Regarding charbono's question about yeast, it's still the yeast that are responsible for the majority of the CO2 needed to make the dough rise, correct?
Great read that was.
I have 2 questions about bacteria and lower temperatures:
1. If starters are frozen and/or kept at a temperature below 38°F, does this kill off the bacteria?
2. If so, when said starter is revived, will the same bacteria strain return or does a new one develop?
Thanks again.
John
John, that's a good question. I have read something somewhere about this, but can't remember exactly where. It seemed like a reliable source to me at the time though. The gist was that Lactobacillus sanfranciscensis is relatively fragile and doesn't survive freezing very well. I don't know about other species. But when the starter is revived, you will be feeding with flour; and flours have a mixture of bacteria in them. Whether you're reviving frozen organisms from the starter, dried ones from the flour, or both, I couldn't say. But it doesn't really matter if the organisms came from the flour to begin with---you're seeding them back in. Since it takes about two weeks to develop a good sanfranciscensis population, it may take you that long to get your starter back to what it was (assuming it was in your starter to begin with). The important thing is to resume the same maintenance routine---that is what really sorts out the population profile.
Thanks for the reply. It appears there are many questions vying for your attention here. Hopefully we will not run you off too quickly...
If you happen across where you read about Lactobacillus sanfranciscensis and freeaing, I would be interested in the link.
Thanks again.
John
...Just spotted this, worth taking a look:
How do lactic bacteria affect sourdough bread?
http://www.nyx.net/~dgreenw/howdolacticbacteriaaffects.html
I had seen this or a version of it on another site at one time and lost track of it, and of course the edited version in The Bread Builders has helped me tremendously in this endeavor. I hope that my article helps you to better understand what is being said in these conversations between Wing and Gaenzle. It's not as technical as his papers are, but it's still pretty technical. I have a handful of Gaenzle research papers in my notebook all marked up with yellow highlights :-) They are a treasure trove of information.
...For that very reason I felt many here would enjoy reading it.
Watching the responses here I decided to run some small tests.
Planning on starting again now that I have formed soemwhat of a foundation.
For each stage and "culture" I'll take photos when I restart.
Shooting for the start of Spring, being in N.E. TN. 'Gooder' airborns. :-)
Kudos, Debra! Really excellent work -- helpful not only in aiding understanding of the microbial miracles behind the bread miracle, but also in allowing us better to sort out the, well, wheat from the chaff?, in what one may read in many books, articles, and internet blogs available on similar subjects.
Am I free to copy the text into a file for my own periodic perusal?
Thanks again,
David
No, wait---It's me :-) What took me so long to get to here?
David, you'll be interested to know that this is the article you found referenced on Scientific Psychic. There's actually a history to it now, I suppose. It came to be written as a favor to Peter Reinhart for his whole grain bread book. He asked me to write the story of the "pineapple juice solution" (as he had named it almost from the beginning), and because I used the terms homo- and heterofermenters in the essay, he wanted me to explain what that meant as well. I wrote it as a separate piece, but both were cut by the editors for valid reasons. The original version is referenced on page 304 as "Yeast and Bacteria."
Not being one to give up that easily, or let all the time and effort I put into it be for nothing, I overhauled it, added the text boxes with diagrams, and turned it into an article. I submitted it to BBGA, and it was published in Bread Lines in Dec of 2007 under the title "Sourdough Bacteria: A Closer Look," but without my diagram of the pathways. I'm not sure if it was a space issue, or if that part just looked too intimidating (but I thought you guys could handle it). Because there was so much interest here, I decided to edit and reformat it once more to publish in this form. All I'm asking is that everyone be ethical.
Onward :-)
Debbie
Hi again,
It took me a while 'cause like you I sometimes get lost in the TFL Wilderness! (And then there's that nasty job of mine that keeps on requiring my time, darn it all.)
I figured this was the article, as allowable by publishers of record, and it's a knockout. I just hope Floyd has stashed it in a place where it won't get buried in the 12 layers of Troy that are always accruing around here! As an Advanced Topic I think it will remain accessible, as is fitting.
I see you have sprinkled considerable wisdom around these parts, elsewhere, and are naturally becoming much in demand. That too is fitting.
Keep up the great work!
David
I'm still waiting to hear something back on the main article. The board meeting was supposed to be this week sometime, and coming up with a policy for these things was on the agenda. I'm hopin' they give me permission to post the pdf, but I really have no idea what they'll decide. If the meeting was today, I may not hear until next week. But I can't complain. BBGA is a great organization and I'm just happy to be a member :-)
And now, life is calling (have to go start dinner ;-)
Debra
Thanks for all the info you presented. I find all the stuff that goes on in the bowl fasinating. I have a couple of questions for you.
1. If your starter is kept in an environment that favors one of the LABs will the other ones eventually die off? I have a starter that raises bread great but is very mild. I have tried feeding it like the books and Fresh Loafers say to produce a tangier bread. I have also tried progressive builds with little luck. If one segment of my starter is gone, should I make a new starter to reintroduce the missing LABs and other "stuff"? I would however need to store it in a different manor to maintain its balance or I would be right back to where I am now.
2. Is a certain volume needed to keep and perpetuate a balanced starter? I keep a small 50g starter to reduce flour waste but would trade flour for the ability to control flavor. It would seem to me the smaller the starter the less stable it would be?
3. What is a good baseline temp and hydration to keep and perpetuate a well balanced starter?
Thanks again for the writeup and answers to my questions.
Da Crumb Bum
1. If your starter is kept in an environment that favors one of the LABs will the other ones eventually die off?
I don't believe that they die for the most part, but rather just fail to thrive under the prevailing conditions. Viable, but not growing or dividing. Waiting kind of like seeds, for more suitable conditions. Some will be discarded each time you refresh your starter, but continuously added back in with the fresh flour. Their numbers will be very low compared to the active organisms, but ever present. Of course, the exception is if they aren't present in the flour, or somehow finding their way back in. Then they could eventually be flushed out. Was it a purchased starter, or one that you created from scratch?
However you have been maintaining it is what has brought about the balance you have now, so that is probably the first thing to take a look at if you want it to change. But keep in mind, that producing tangier bread may have more to do with what flours you use and how you manipulate the dough, than how sour your starter is.
2. Is a certain volume needed to keep and perpetuate a balanced starter?
Yes and no. There are so many organisms in even a speck of starter, that size isn't much of an issue. Except with respect to temperature. If the air temperature fluctuates quite a bit, the temperature in the starter will be more stable in a moderate or large volume than in a small one. But at the other end of the spectrum, microorganisms do generate a little heat, so large volumes may actually retain heat and get warmer than the surrounding air temperature. Temperature does make a difference to stability, as does a regular feeding routine.
3. What is a good baseline temp and hydration to keep and perpetuate a well balanced starter?
There is really no right answer to that question. I highly recommend reading Daniel Leader's Local Breads. In this book, he takes a journey through various regions of France, Italy and Germany, to learn the secrets behind the different styles of local breads across Europe. He brings it all together---the way the starter is maintained in each region, characteristics of the flours, the process, and the resulting character of the bread produced from them. He doesn't talk science, and yet it all makes sense from the scientific perspective. He really brings it to life. It's my new favorite bread book :-)
And it really kind of leads you to see how all these bread processes evolved to make the most of the flours grown in their respective regions, as really all cuisines evolve around local ingredients and culture. Recently there was a thread kind of trashing American flours compared to French. But can you make a fair assessment swapping one flour for another in a process designed for a different type? The flours are different, that is true. But if American flours are so inferior, I'm not sure how the USA came away with so many gold and silver medals at the Coupe du Monde competitions in France over the last decade or so. Placing higher than the French, more than once. Those guys know their flours and how to bring out the best in them. After all, you wouldn't use a Bordeaux grape to make a Chianti. Just food for thought...
And Eric, don't give up on me yet---I'm not forgetting you :-)
Debra, you wrote:
But if American flours are so inferior, I'm not sure how the USA came away with so many gold and silver medals at the Coupe du Monde competitions in France over the last decade or so.
The answer is quite simple:
1) All Coupe de Monde contestants are required to use French flour and
2) Even if the U.S. teams that won gold did use U.S. flour, they probably still would have won because they were superior bakers. They had the knowledge and skill set to work with whatever type of flour put in fromt of them to produce better loaves.
I think you might have mischaracterized the recent U.S./French flour thread as 'trashing' U.S. flour. The message I got was that when using U.S. flour, one could achieve similar results to those obtained using French flour but it required a bit more effort (at least I found that to be the case).
SteveB
http://www.breadcetera.com
SteveB, I was under the impression they took their own ingredients. Thank you for setting me straight :-) I'll correct my post.
Well, I tried to edit the post, but it will no longer let me. My appologies for the mischaracterization.
Best,
Debbie
Debra
Thank you for answering my questions. The starter in question is one I started using grapes from a friend and following PRs Crust and Crumb directions. I really like it and it raises bread very well. It has evolved though and I was curious about bringing it back to how it was or just being able to manipulate it for desired results. I agree that a larger starter would be more stable temp wise. I will also try Local Breads on your recommendation. This stuff is fascinating and I only really understand the top of this "iceberg" of info. At the end of the day its flour,water,salt and starter in a bowl but after reading your post it I will think about it all differently. Thanks Again
Da Crumb Bum
Debra,
I have been following along in this and other threads you have been participating in and enjoying your thoughtful replies. Thank you for your patience in answering what must be to you, very basic questions.
I want to ask you about using a blend of rye and whole wheat with AP or bread flour for feeding. A while back I read I think it was Dan Lepard saying how he suggested feeding a blend of 70% White Bread flour, 20% Whole Wheat and 10% Rye. I tried this blend in my already active starter and it nearly exploded. The results were quite amazing in terms of increasing the activity. What happened was that it became such a voracious consumer that if I skipped a feeding it was wasted and a slack, sticky mess within 24 hours. I didn't notice a big change in flavor but I was getting a nice open crumb and a mild sour.
I stopped using that ratio and have since backed down from the blended flours and now use a pinch of rye in the feeding stock of AP on occasion.
If I understand what you have said above, I should be able to develop a L.sf culture from my now stable starter by establishing a room temp (65-70F) feeding regimen using straight bread flour. From the standpoint of the yeast population, adding a small amount of whole grain flour in the feeding should encourage the yeast growth, yes?
I have been placing my starter in a 75F spot during the wake up phase of elaboration, after a feeding. I get an active looking culture but I guess that is just the yeast performing. If I were to use a cooler location, say on the counter at 65F, I should get a higher population of LAB or the sf strain in particular.
I'm not sure I have asked you a clear question in all this. Sorry! My driving passion is to make the best possible smelling and tasting SD bread. Gaining a better understanding of the make up of the culture we call a "starter" should hopefully help.
Is there a way to get an analysis of a culture to discover what the various populations are and at what percent? Maybe a college science lab? I might be up for a one time test just to see.
Thanks again Debra,
Eric
Thank you for your patience in answering what must be to you, very basic questions.
Actually, I've been really impressed with the overall level of knowledge and comprehension here :-) I like the opportunity to answer questions, because it helps me sort things out and make new connections of my own. I'm just sorry that I can't always get to them immediately.
using a blend of rye and whole wheat with AP or bread flour for feeding... if I skipped a feeding it was wasted and a slack, sticky mess within 24 hours
One of my most memorable lightbulb moments was sitting in Dave Miller's whole grain breads lab at Camp Bread 2005. He put up a cut-away diagram of the anatomy of a wheat kernel with all the parts labeled, and pointed out where everything was located. Not just bran, germ and endosperm, but the other substances in and on the seed---bacteria attached to the outer bran layers, the bulk of the enzymes located just underneath the bran, etc. And he talked about the parts that get stripped away in the processing of refined bread flours.
I had never really thought beyond the bran and germ before, in terms other than the fiber, vitamins and healthy oils that are lost. But bacteria, and natural enzymes are also stripped away. I wasn't quite grasping the significance of this, but the epiphany finally came when he summarized: Everything happens faster with whole grain. Those were probably not his exact words, but that was the meaning that hit me. Such a simple statement, and yet it explains so much. About why whole wheat doughs get so sour and break down with long fermentation---at least mine always did. In that moment, I understood as if I'd had some sort of spiritual awakening. (Don't laugh.) All the over-fermented desem doorstops and too-sour whole wheat breads suddenly made perfect sense. No wonder whole grain breads are so challenging. The flour may be wheat like bread flour, but whole grain is a whole different animal.
Since then I've learned that pentosans and FOS (fructose-containing complex carbs) reside mainly in the outer layers as well. Not only that, but among the enzymes are aspartic proteinases---proteolytic enzymes that are pH-activated. So, as a result of natural enzymes liberating sugars to the organisms more quickly for fermentation, the acids generated by the organisms in the process, increase the action of proteolytic enzymes. And poof! If you miss a feeding, those enzymes can gobble up the gluten like little pac-men. That translates into a slack and sticky mess.
If I understand what you have said above, I should be able to develop a L.sf culture from my now stable starter by establishing a room temp (65-70F) feeding regimen using straight bread flour.
L. sf. needs an environment that doesn't remain too acidic through underfeeding. That said, the literature I've seen on this only looked at starters maintained at what I would consider to be warm room temperature (25C/77F), or higher. I think maybe this is closer to what the ambient temperature might be in a bakery where ovens are operating all day. Homes are quite a bit cooler on average this time of year. I would expect it to do okay at 65-70F, as long as it gets fed everyday. (But I have no data to back that up.) It wouldn't need to be fed as much as one kept 10 degrees warmer, but still enough to control pH.
From the standpoint of the yeast population, adding a small amount of whole grain flour in the feeding should encourage the yeast growth, yes?
That appears to be the case from the reaction of your starter, but I'm sure the LAB are getting a shot in the arm too. The increased enzyme action of the whole grain is breaking out more sugars they both can use. But something else to keep in mind is that yeasts and L. sf. can only use the 6-carbon sugars; neither can utilize any of the 5-carbon sugars that rye and whole wheat bring with them. That provides an additional food supply that another heterofermentive LAB such as L. plantarum can tap into. When pentoses are being metabolized, acetic acid is produced. So, 5-carbon sugars will probably have an effect on population dynamics and contribute its own influence. I'm not saying that's good or bad, that's for you to decide.
I have been placing my starter in a 75F spot during the wake up phase of elaboration, after a feeding. I get an active looking culture but I guess that is just the yeast performing. If I were to use a cooler location, say on the counter at 65F, I should get a higher population of LAB or the sf strain in particular.
Just the opposite actually---but then, you've probably seen my response to Pat about L. pontis since you posted this. That's where the answer is. 75F is great as long as it is fed often enough.
Is there a way to get an analysis of a culture to discover what the various populations are and at what percent?
Unfortunately, no. Well, someone could do an organism count, but they wouldn't be able to ID these kinds of species for you beyond yeast and lactobacilli. The problem is that many of these require very sophisticated methods, including DNA analysis to sort them out, that ordinary labs don't do. The only labs identifying these lactobacilli are the labs doing research, and even they experience challenges from one lab to the next. This was actually the subject of an article by Luc De Vuyst and Marc Vancanneyt, titled Biodiversity and identification of sourdough lactic acid bacteria (see my bibliography section).
Whew! That was a long one :-) Have I missed anything, or anyone?
Debbie
Thank you for hanging in there and thoughtfully answering my questions and the others that were presented. Since I have been hanging around TFL we haven't had someone with your credentials to speak to the sourdough science. I fully realize that our mileage may vary but I know I have already seen a change in the personality of my starter and thus my breads since I adopted some of your advice.
For example I decided to stop treating my starter culture like a bucket of yogurt and to keep it at room temperature, feeding in the evening. So I am mixing a daily pain au levain using a 50g inoculation to 500g of flour with 5% of that being whole rye. The mix sits at room temperature overnight and gets baked the following afternoon. My breads are getting increasingly sour. This is a good change for me as we like a moderate sour. I haven't checked the pH of the starter but it has a very nice wine or cheese aroma and triples in 6 hours. The feeding ratio has been 1:1.5:1.5. I'm starting with 50g of starter and feeding 75g of water and flour.
Debbie I am detailing my results so that others who may read this thread will appreciate the value of room temperature culture maintenance and be able to gain some insight from my new wisdom. Please don't think you need to reply to my details, you have done enough as is.
Thank you again Debra,
Eric
What type of flour are you using to feed your starter?
If you were to only bake once a week, would you still maintain it this way?
Where do your discards end up?
I would like to try changing how I am maintaining my starters just to achieve more sour...I want it real sour.
Thanks,
John
John,
I have been using Gold Medal Better for Bread as my stock bread flour and feed stock for my starters.
If I were going to bake just once a week, I think I would start feeding my starter at 100% hydration, once a day at the same time and use the same temp water every day. I would continue to follow this routine for at least two weeks, maybe longer. The regularity of feeding I think is important and you need to find a routine that you can stick to at some time of day. If after a couple weeks your bread develops a nice sour after aging 14-16 hours or overnight in the cooler, maybe you could start keeping it refrigerated at a lower hydration using a weekly feeding schedule or even longer.
I have had good luck using 60% hydration and 10-14 day feeding cycles before my recent enlightenment care of Debra Wink. Keep in mind I'm talking like I know what I'm doing but in fact you are only a week or so behind me in this. My breads have always been good but not overly sour regardless of what I do to make it otherwise. The last two days my breads have had a much better tang without seeing the inside of a cooler. Just long fermentation at room temperature.
Please let us know how it turns out. I'm very interested in knowing if my experiences are repeatable elsewhere. The starter I am using now is one I created about 6 Months ago using the instructions in Bread by Hamelman. It was easy to get going and seems impossible to kill. Good luck!
Eric
I too have been leaving my starter out at room temprature since the end of December. The bread that I'm now making is just right for me. To me it reminds me of a sourdough bread that is made near here. The bread is full of flavor too. I want to run my test a few more weeks to see if I'm really on to something here but I'm really happy with the results so far.
"My breads are getting increasingly sour. This is a good change for me as we like a moderate sour."
Ahhhh... this is what it's all about---empowering people to make informed decisions that get them closer to creating the kind of breads they want :-) I think this new understanding will help you (and everyone that you help) to problem-solve more effectively. But I'm sure you'll agree that making the process a little less mysterious, makes it no less wondrous.
Debbie,
To me, any time I have to take the whole of my knowledge on faith, it's a mystery. I would love to be able to look at these critters with my own eyes and see the changes I am trying to make. Sadly we are left with having to eat our work and sort it out over wine and cheese or lunch meats.:>)
Eric
I'll cry to that! "Pass the butter!"
Mini
Hi, Eric.
It doesn't have to be a mystery. You just need the right equipment. Every kitchen should have a scanning electron microscope!
Notice that this model includes a bench for kneading and shaping your loaves.
But, be assured, this in no way replaces the need to eat your work.
David
"we are left with having to eat our work and sort it out over wine and cheese..."
Well... that's just not sad at all ;-)
Here's what mine looks like under the light microscope. Or at least it did in 2002, when I took this photo using a setup similar to the one in David's post. I'm sure yours would look much the same. While it's kinda cool to see the critters in action, you still have to eat your work to detect the changes. But that's not really so bad, is it?
Is there anyone here who can label stuff in photos like this for me, so that I can point out the yeast, LAB, etc., and post these in a blog about making sourdough starter? I got the blessing from BBGA to post my article, as long as I don't use their pdf, so I need to relabel the photos, and I'm not that technologically advanced. You're welcome to let me know through the message feature. TIA -Debbie
The big, round critters are yeast. You can see some of them budding. The long, skinny, pale dudes are bacilli, presumably of the Lactobacillus family. Most of the round stuff with thicker walls are most likely junk - bubbles or artifacts.
David
Good effort! What you have labelled as Yeast are actually starch granules (flour). Bacillus is a different genus of bacteria entirely. And, no, those aren't bubbles. Thank you for playing Name That Critter ;-)
I'm just teasing ;-) A graphic arts person has come to the rescue, so hopefully, we can get this done. Thanks again---I really do appreciate the help.
Debbie
Hi, Debbie.
I wondered about starch granules. But, I figured the relative size of what I identified as yeast and bacteria was about right. I eagerly await the corrected test.
David
Right smack up from the middle, little round things, they look like organisms. And LABacteria, I would guess they were the white shadowy little bits of tiny line like things. All those little clumps of things...of uneven shapes and sizes (that remind me of patio rocks) are the yeast. (How am I doing?) You already said the big round things were startch... so that's what food looks like to a wee beastie! Glad they don't have eyes...
Mini
I really thought you had it until you said the patio rocks are the yeast ;-)
You are both right in that the really tiny shadowy things are bacteria. Two kinds of LAB in this photo---lactobacilli (the rods that David labelled Bacillus, even though I know he didn't mean the genus) and probably a Weissella (the tiny spherical ones).
"Right smack up from the middle, little round things, they look like organisms."
Good eye. They are organisms. Those little round things with a crinkly-looking texture. Those are the yeast :-) Everything else you see comes from the flour.
Mini O
I recall when I first looked at an ultrasound, and the doctor said "look you can see her head". I nodded in agreement as I stared at the fuzzy image that had no apparent form. I feel like every time I look at the image above I see a better level of detail. There are a lot of small distinguishable details if you look closely.
Eric
I have just finally read this from start to finish - sort of grasping some of it - and wanted to add my thanks for an exceptional article.
Sadly my somewhat limited grasp (aka virtually non-existent) of microbiology and organic chemistry leave me struggling with two phrases you use:
co-metabolism and co-substrate
Could you explain in simple language or point me in the right direction to to a resource that could help me understand these terms. I feel like I'm missing out on at least 50% of the article without this understanding.
Also, looking at the hetero and homo fermentatitive pathways for fermentation of glucose, I am somewhat confused how a hexose can be split, in the heterofermentative pathway into 2 and 3 carbon pieces? 2 + 3 = 5 surely? Or is one glucose split into 3 x 2 carbon while another may be split into 2 x 3 carbon? Or, as I suspect, I am completely misunderstanding the diagram?!
Many Thanks,
FP
Let me start with the easier one:
What happens with the hexose, or 6-carbon sugar, is that it first needs to be turned into a 5-carbon sugar to fit the heterofermentative pathway. One of the carbons is removed by turning it into carbon dioxide (CO2), a 1-carbon molecule. CO2 is "cast off" to get rid of extra carbon. So you'll find the missing carbon there between 6-phosphogluconate and ribulose-5-phosphate. And you can see on the diagram, that CO2 is also cast off on the portion of the pathway that represents alcohol fermentation by yeast. In that case, a 3-carbon molecule (pyruvate) is being cut down to a 2-carbon molecule (acetaldehyde).
Co-metabolism is a term that means two things are being metabolized together. In bread, most often those two "substrates" are maltose and fructose. Both happen to be 6-carbon sugars, but only the maltose is used for its carbon backbone. That's why it is refered to as "the carbon source." It's the one that is processed and broken into 1-, 2-, and 3-carbon pieces. The co-substrate---fructose---is used as sort of a helper molecule, in lay terms. Without it or another co-substrate, the 2-carbon piece will go on to become ethanol. Fructose, when present, facilitates the alternate end-product formation of acetic acid. It does this by acting as an electron accepter, and I'm sorry I don't know a lay term for that. But, it allows acetyl-phosphate to become acetic acid (and in the side reaction, fructose is changed into mannitol).
If that doesn't clear it up, just ask me a new question :-)
dw
Thanks for that reply - that makes sense now. Thankyou!
However this does raise one question...or ponderment really. If alcohol fermentation by yeast uses the pyruvate coming through the 3-carbon pathway then increased yeast activity might slow the build-up of lactic acid?
Also - is it possible to add particular sugars (eg maltose-rich malt syrup) to bread dough in order to encourage the growth of and influence the metabolic behaviour of particular lactobacilli?
For example, it has been my lay observation that malt extract (not diastatic) added in small quantities seems to make the final bread more sour. Rather naively, I assume this is because lactobacilli such as lb sf prefer maltose while yeast such as candida milleri do not...I'm sure this is a vast oversimplification of what might be going on (you mentioned glucose excretion by lb sf for example). It's possible that my observations merely coincided with some other variable or fermentation factor. Again, I look to your expertise to cast light on this.
Thanks again,
FP
Competition for pyruvate isn't an issue, because everybody makes their own, and this is going on inside each cell, where other cells don't have access to it. Yeast follow the EMP pathway from glucose to pyruvate, the same as homofermentation, but it's what they do with the pyruvate that is different. Yeast turn it into alcohol and CO2 when there's no oxygen available, and LAB turn theirs into lactic acid.
My diagram is a universal "map" of all the fermentation pathways mentioned in the article, as if it were transparencies of all the different routes stacked on top of one another. Included is alcohol fermentation (yeast), homolactic fermentation, and all the different variations on heterolactic fermentation (hexoses, pentoses, with co-subrates, without...). The one part shared by all is the 3-carbon leg from glyceraldehyde-3-phosphate to pyruvate. I wanted to show the similarities as well as the differences in a more visual/conceptual way.
Yes, you can add things to dough to influence end-product formation---they do it in industry. Increasing maltose in the dough could certainly increase activity of lactobacilli, which would increase lactic acid production. To increase acetic acid, however, you'd need to increase co-substrates in the dough (fructose, malic acid, citric acid, pentoses, etc.). One way to increase fructose is by adding sucrose (white table sugar), which is a disaccharide that is half fructose. Honey, is a mixture of glucose and fructose. You'll have to decide which additives are acceptable to you. Do you have a philosophy regarding your bread?
I think the artisan movement leans more toward coaxing all these things and more out of flour with water, salt, leaven, time and temperature. Probably, everything you need can be found in one flour or another. Especially whole grain flours which are good sources of inulins and FOS (fructo-oligosaccharides) for fructose, pentosans for 5-carbon sugars, and natural enzymes to help unlock them. Many bread formulas include a small amount of rye or whole grain. Try out a variety.
But yeast have to eat something though, don't they. Maltose is the most abundant food source, so yeast are benefiting from it, whether they can directly utilize it, or they rely on glucose the lactobacilli spit out. I'm not sure if there are enough other sugars to sustain them (unless you add another sugar).
As a side note, I haven't seen Candida milleri mentioned in the more recent literature of the past 5 years or so. The yeast most commonly found in type I sourdoughs is Candida humilis. I suspect that there was a reclassification somewhere along the line, but I haven't read any statements clearing up the disappearance of C. milleri. Both seem to still be separate species, but sometimes a strain will be moved from one species to another. This has happened a lot since the advent of DNA analysis. (The old classification systems were limited to phenotype.)
Again, thank you, Debra. You are truly a fountain of knowledge.
I agree with you regarding additives. My philosophy, if it can be called that, is to try and get the maximum from the flour first before looking to additives. However, understanding why and when they might be appropriate helps me appreciate the overall fermentation process better as well.
Thanks again,
FP
Philosophy wasn't quite the right word, but I couldn't think of another. We all have our own reasons for baking bread, and our own criteria for the food we choose to eat. I'm not against additives by any means, but I'm not in favor of relying on them merely as a shortcut. To borrow your phrase, "understanding why and when they might be appropriate," in appreciation of the overall fermentation process, is the key to improving bread vs cutting corners.
dw
The vast majority of people won't have any interest in this, but it relates to what I wrote originally in this article---about Lactobacillus sanfranciscensis fermenting glucose poorly and fructose not at all. It also speaks to the popular (mis)belief that maltose is the only sugar this organism can utilize. After reading an article by Spiros Paramithiotis on 'Flour carbohydrate catabolism and metabolite production by sourdough lactic acid bacteria,' my understanding has changed a bit, and so I felt I needed to do a slight revision.
In the study, Paramithiotis et al., isolated various lactic acid bacteria (LAB) from traditional Greek wheat sourdough, and grew them separately in liquid media containing glucose, fructose, maltose, or sucrose---as the single food source and in combinations---to see what effects these have on cellular growth and metabolite production (lactate, ethanol, acetate and mannitol). They demonstrated that L. sf. (strain ACA-DC 3366) can utilize all but sucrose. While maltose produced the fastest growth rate and highest final cell mass of the four sugars separately, L. sf. reached the same cell mass with glucose, when fructose or maltose were also present, even though the growth rate was slower. And optimum growth was achieved only when all carbon sources were combined.
When fed only fructose, L. sf. not only fermented it (used it as the carbon source), but utilized it as electron acceptor as well, producing lactate, acetate, and mannitol in roughly a 1:1:2 ratio. (As electron acceptor, it gets turned into mannitol.) But when fructose is present in combination with another fermentable sugar, it is preferentially used as electron acceptor (co-substrate) rather than as carbon source.
What was surprising (to me) was that with maltose alone, there was no extracellular accumulation of glucose---the organism used that too. Glucose by itself, was almost completely converted to lactic acid and ethanol, indicating that it was used to generate energy. When glucose and maltose were present together, they were consumed at the same rate, but only maltose was completely converted to lactate and ethanol. The glucose then, must have been diverted to alternate pathways and/or end products not measured in this study.
All that said, only one strain of L. sf. was represented in the study. In contrast, other papers such as Michael Ganzle's, on 'Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough' states:
So, Paramithiotis' strain appears to be atypical for the species, but it does help to illustrate just how much variation there is, and how the organisms alter their metabolism to fit their needs in changing environments. If they run out of maltose, they do the best they can with what's available.
It's just never that simple with living things,
-dw
There is such a wealth of material to be mined in the archives here, I have to use the timer! Despite my great interest in learning about sourdough starters and breads, I hadn't made it to this thread previously.
Thank you for the accessible manner in which you have presented this information, although I majored in biochemistry and took microbiology too, prior to venturing into the world of TFL, a long time has passed since I drew on that. (Must say I'm always a bit fascinated by what can possibly be going on in the microworld of my worm farm!)
Following your encouragement to test things for ourselves over the last couple weeks, with our daytime temperatures at last reaching what tends to be called room temperature in USA bread publications, and my concern about underfeeding my starter using the refrigerator maintenance recommendations in the TFL Handbook (there were so few responses to my separate query in the Handbook Forum in that regard and I'm so new to this I haven't been able to make a recommendation....), I have been storing my starter on the bench in the kitchen and feeding it twice a day. I tend to alternate between making a multigrain sourdough (playing with the grain combinations in the soaker) and something new (so many breads, so little time). Have only done a couple of multigrain bakes with the 'well fed warm' starter, using grain combos used previously and found the resulting bread to be sweeter ( I don't like this sweetness) but I plan to persevere and see what happens with time. I'm also going to put some back in the fridge to use in some side by side bakes. Not only is the bread itself feeding me, but the fun of altering the parameters is sustaining me too.
Cheers, Robyn
It's good to know you were a biochem major. Between you and SteveB (organic chemist), I can get my chemistry questions answered. I was the other way around---majored in microbiology and took biochemistry, though I have no fond memories of that ;-) Of, course it was so long ago, that I can't remember much of it at all.
Whole grains are naturally sweeter than refined---are you wishing to increase the sour?
Got here because your update triggered my RSS reader -- and have spent a happy half hour half remembering undergraduate biochemistry. A wonderfully informative post.
Jeremy
Thanks guys! Hope you'll find the info to be useful.
Happy Baking,
-dw
Dear Debra
I have to say that I'm really thankful for your time and your effort to post this imformation. I got microscope too and I'm really interested about micro organism. But it it not easy to understand especially the chemistry form. which I don't have a clue. I saw exactly same as your picture in my microscope, but I can't tell which is what.
Anyway, thank you so much.
Laddavan Charoensuk.
If you can distinquish the yeast and bacteria from the rest, then everything else is just flour. There are two types of starch granules:
Bran particles look more irregular or ragged. You'll definitely see them if there is whole grain flour in the mix, but not so much in highly refined flour. There are no gluten strands in my picture, because they tend to stick to the loop, and not float off into the water drop with everthing else. Hope that helps :-)
-dw
Edited to correct a typo (sherical changed to spherical)
Oh my goodness. I asked my wife who is a MD retired and she misunderstood me. Then asked why was I intersted at my age. Just to add a little humor to the post.
Mr. Bob
Yes, I saw some think float also :) Now I slowly learn. :b
Laddavan.
Thank you so much. I saved this information to read again. :)
Laddavan.
I must have missed this very informative exchange because when the thread started, I wasn't into sourdough breads then. I'll be going into sourdoughs next year and in fact started my starter last week. I will bookmark this exchange as a valuable reference tool.
Thanks so much for sharing, Debra!
Thank you. Thank you very much for all the informations you gave to us. Now everything is more clear.
There are 2 things that I would like to ask.
1) You say:
"Additional energy can be produced by turning the 2-carbon piece into acetic acid, ........... The co-substrate can be ............. fructose and 5-carbon sugars. In the absence of co-substrates, the 2-carbon piece is turned to ethanol instead. Alternatively, when pentose sugars are fermented (used as the carbon source), acetic acid may be produced without the help of co-substrates."
So, HOW pentose sugar can be fermented WITHOUT CO-SUBSTRATE? And it happens always, when pentose sugars are fermented?
2) Why sometimes bread is RUBBERY?
After I leave the bread in a bag for one or two days, the bread, that was crispy, is rubbery. Especially if the flour contains a lot of gluten. WHY?
And if I defrost bread with microwave oven (instead of using gas oven) it is rubbery (but the bread is less gummy if the bread contains hard wheat flour, like ciabatta bread that they use in Siciliy). WHY?
I hope you have time to answer, and thank you, thank you very much again for your wonderful article.
Giovanni.
I wish I could give you a definitive answer on #1, but I can't seem to locate the quote that would best answer it. I'll keep looking. I remember the gist was that they didn't know why, but theorized that it had something to do with the regeneration of cofactors (less NAD is used up in fermenting pentoses).
I know that's not a very complete answer, but that's the best I can do until I understand it better myself.
Question #2 is out of my area, but you might get an answer if you pose it in a new forum post to everyone. Good luck :-)
Thanks again!
-dw
HI, maybe you are talking about ciabattas made with durum wheat flour, the yellow one that is used to make pasta. Hard wheat is what in italy we know as manitoba (yes, very confusing). What flour gives you the most gummy bread? I guess manitoba, that is much richer in gluten. Generally the solution against gumminess is simple: use less yeast (1% of the weight of flour, so 1 gr of fresh yeast for 1 KG of flour) and make it raise for much longer time, or use a sourdough that is slow on its own accord.
Also, keep in mind that weaker flours (such as durum and soft wheat) won't bear such a long fermentation (especially durum wheat tends to disintegrate), unless they are enriched with vit C. You should make some test and see how long time your flours bear fermentation.
Thank you Debra, you are so kind. I hope you will find something, but I know it's not so easy, and if I'll find something more about the production of acetic acid without co-substrates I tell you too.
Thanks to nicodvb too. I'll post the question of rubbery bread in another post, to ask what is the mechanism that transform a crispy bread in a rubbery bread after some hours or days.
Another article you can look at is Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough, by Gänzle, et al. It shows how NAD is regenerated when a co-substrate is used (specifically fructose, oxygen, oxidized glutathione or an aldehyde). Maybe that is the co-substrate's real function in the process.
When a 6-carbon sugar is fermented, an extra NAD is consumed (before the CO2 is kicked out), which is ordinarily regenerated when acetyl-P is directed down the path to ethanol. But the pathway to acetate production is preferred, since it generates an extra ATP of energy. And the co-substrate helps to balance the books, so to speak.
Because fermentation of a 5-carbon sugar by-passes that first part of the pathway, it wouldn't need to regenerate the extra NAD. I think that is the presumption anyway. These articles are all a couple of years old, so maybe more is known today. And, sometimes we have no choice but to be patient and wait for science to catch up. Is this is a pressing issue? -dw
Thank you Debra,
I'm looking for more tedails, but it's not urgent at all: it's just the pleasure of the knowledge, to understand how and when they don't use co substrates. I'll tell you if i find more.
But everything now is much more clear after your articles.....
Giovanni
Thank you so much for all the posting you have done on TFL, Debra, I have gone back to your posts over and over again as I work with my sourdough starter. With the cold front we had last week here in Thailand I was excited to see if I could stimulate some yeast/lactobacillus growth, and the results were not what I expected at all. I was using the temperature chart from Ford:
http://www.thefreshloaf.com/node/10251/starting-starter-sourdough-101-tutorial
Yet my dough was incredibly sluggish. All I was able to bake that week was mushy bricks, my bread didn't want to rise after hours and hours of waiting, and I was concerned about overfermentation. Worst bread I've ever made. My starter was sluggish, too. Now it is warming up again and my starter is very active again, so active it is doubled in about 4 hours. Today I found this on my search for answers:
You wrote: "Contrary to popular belief, all three groups of sourdough lactobacilli prefer wetter doughs a bit on the warm side, many growing fastest at about 90ºF or a little higher. For the homofermentive species producing only lactic acid, increasing activity by raising the hydration and/or temperature will increase acid production. Decreasing activity by reducing hydration or by retarding will slow production."
So, as you can imagine, I am wondering what it is that I am growing here and why were my breads failing at lower temperatures? My starter is 100% hydration, 13% protein bread flour.
I realize you are a very busy person and may not be able to get back to me, but thank you again for all the help you have given us here on TFL.
I have used this post many times now and thought it would appropriate to say thank you for taking the time and effort to do it and to answer questions with such useful detail. I don't understand well enough the pathways, atoms and such things as ferment 6-carbon sugars, but I have made use of the rest and will be referring back to here in the current post I'm writing. Between this and other text books I've read and experience of my own with my starter it's falling into place.
Click here: Life Cycle of a Sourdough Starter Part I I stumbled upon the blog post a couple weeks ago, and I've been wanting to go back and say thank you. Just been so busy, and forgot. You're very kind, thank you. Nice work, btw :-)dw
Hi Debra,
Superb!
Thanks so much for taking the time to explain so lucidly what's happening under the hood of our sds. Been looking for some time for a good article that describes the science. Silly me... should have know to look in TFL first. Can't tell you how much I enjoyed the read and following posts...
Hi Debra
Happy to ready you approve of the references on my post. yay! My aim is to "untangle" sourdough and if I can understand it so can anyone else.
I have a real hate for when bakers love to make sourdough so complicated it puts new bakers off.
Information on SD is out there but in bits all over the place and as you know the baking textbooks haven't really caught up properly either, making it difficult to understand properly.
I'm grateful to Daisy for pointing this work of yours to me. It was a breath of fresh air to have so much rubbish that is believe about starters demystified and put into facts. I only heard last month an experience baker teaching a class that if you start your starter with raisins it will likely die on you 3 months later unless you re-introduce it to raisins again! Urmmm....no I said...my starter I began with raisins year ago is a 'healthy bouncy' starter, never misbehaved.
I have many other examples of rubbish said out there. I shall be forever grateful for your work. :)
azx
Hi thanks for explaining the microbiology so well, can you tell me for how long the enzymes do there work to set the stage for the bacteria to take over? I mean how long before the enzymes are no longer the main organism decomposing the flour?
Read for further sourdough development: www.thefreshloaf.com/node/51434/pure-lactobacillus-sanfranciscensis-culture-or-powder-anyone-please
Hi Debra,
Well, this was exceptionally interesting and I have already read it several times. It will probably take many more before fully understand it.
I find myself wishing you had a similar write up specifically on yeast growth in sour dough culture. I know these things are symbiotic, so one can not be mentioned without the other. But I'm specifically struggling with getting good rise out of my starters. The sourness is there in abundance, but the yeast seems to be struggling.
I've gathered tidbits from your posts and from others who are clearly very knowledgeable, but sometimes they are contradictory or if they are not, the nuances as to why the advice is varied but still accurate is lost on me. (Most of the literature out there seems geared toward brewers.) For example, in this post, I understand that yeast tolerate lower temperatures than LAB. But in a grid dabrownmen shared (which I can no longer find), the y/lab ratio is highest at higher temperatures. Needless to say, I'm a little confused about how to tip the scales back in favor of more yeast. I like sourness, so, I'm not trying to eliminate it, but if my dough won't rise, well, it's not a great bread. I also gather from your post that a stiffer starter would favor yeast over LAB.
It would just be great to have a comprehensive understanding of yeast behavior in starters and since you've done such an eloquent job with LAB, I wonder if you have written something more focused on yeast somewhere or if you can point me to a resource. (I have also read your pineapple juice solution.)
Thanks again! I'm always amazed at the willingness of bakers to spend so much effort sharing information out of the goodness of the hearts.
Hi BB, and welcome
Don't be too concerned with those temperature charts, they really don't have much practical application in sourdough culture. Growth rate charts generally come from growing pure cultures in laboratory media under a specific set of (otherwise ideal) conditions that are meticulously controlled. Mixed cultures growing in sourdough are a different ballgame, and the relative growth rates are influenced by the sum total of all factors, known and unknown (including how much antagonism there is between the populations) -- Not just the temperature. Sourdough is actually a difficult environment for the microorganisms. Also, when LAB are high, they have a suppressive effect on yeast. Growth rate charts don't reflect that, or tell you how long the lag phase is for each population after refreshing under the prevailing conditions. That is far more important, and therein lies the problem. It's never that simple with living things.
Moving the maintenance temperature up shifts the balance toward LAB, and moving the temperature down will favor yeast over multiple refreshments relative to where you started (all else being equal --- always the qualifier but seldom the case in bread-making). Either may or may not give you the flavor profile you like. You just have to try it to see. I find an easy way to increase leavening power and control sourness is simply to feed more often. If you're only feeding once a day, step it up to every twelve hours. If you're already feeding twice a day, then add a third on the day you're getting ready to make your preferment (or any time your starter needs it). The feedings don't have to be equally spaced, but in any case, aim for a refreshment rate that gets the starter to the next feeding still in the window of ripeness, not deflated. Allowing it to chronically over-ripen will undo what you're trying to accomplish. Besides cooler temperature and feeding more often, maintaining the mother culture at a lower hydration works well too --- again, not letting it deflate too much between feedings. Try just one change at a time, and give it time to adjust. It may take a few days to really see a change.
Here is a post that may help you understand why ripeness and feeding schedule make a difference. The whole thread has a lot of good info if you have the time to wade through.
http://www.thefreshloaf.com/comment/116222#comment-116222
Thanks for you question.
My best,
dw
Yes, your answers here and that thread were exactly what I needed to help build my understanding. I've been close to losing my mind over this stuff. What a relief to gain some clarity.
I've been searching and searching for some sort of definitive text on sourdough cultures, but I think the most useful information is right here on your posts. I'm sure others have implored you to write a book. I'm adding my voice to the chorus. I could see it being an immediate classic, sitting on shelves between Hamelman and McGee. (Do you have an index of your posts somewhere? Searching is possible, but not very easy here and every time I find a nugget from you I feel like a prospector shouting "I found gold in them there hills!" I know some things would be hard to share out of context of a particular post, but an index of links or something would be awesome. If not, I might just make compiling that my own personal mission.)
Most bread books have about 3-10 pages on this stuff. These are very fine bakers but the problem is they are drawing on their own practical experience and applying scientific rumor to explain the results they're seeing -- yeast captured from air; acetic acid production increases at lower temperatures. Of course their experience is extremely valuable but it doesn't help very much when a reader following their advice needs to troubleshoot a problem or doesn't understand how the conditions differ in their own home environment. Muddling scientific rumor with different results in the home kitchen create a difficult gauntlet to navigate. To escape the maze, we need a backbone of real, scientific understanding. What you are doing is extremely difficult. (Perhaps that's why I cannot find anybody else doing it). You are providing that scientific backbone to laypersons while not bogging us down with too much detail that would lead down a scientific rabbit hole to a place of no practical benefit.
Thank you. I have new questions, but I'm going to spend some time studying the great content you have out there and chew on it before I ask.
Your comments are appreciated. I do get the book request more and more. Unfortunately, it won't happen overnight. I don't have an index of my posts, but if you scan through comments of the big four --- pineapple juice solutions 1 & 2, lactic acid fermentation, and very liquid sourdough threads you're already familiar with, I have put a lot of links to other things I've written in there. You can also go to my profile page and click the track tab, although that will bring up every post I've responded to over the years --- many having nothing to do with science. But the subject lines might help ...
Hi Debra,
I’ve spent a lot of time studying your posts and I’m ready to make some adjustments to my own sourdough culture based upon your advice to others. Specifically, I’m going to be switching from 100% rye to a majority of white flour and making the starter more firm. I understand I need to make one adjustment at a time.
You’ve said that it takes several feedings for the microbes to select for the new environment. Makes sense. But are those feedings used to gradually adjust the environment, i.e. slowly increase the percentage of flour over multiple feedings until arriving at the new percentage goal? Or should I go straight to the new environment and feed it immediately with the new percentage of white flour several times before using it?
I expect it would work either way, but which is the most effective and quick? Let’s assume I don’t care about having viable starter for raising bread in the interim.
Thanks!
Whenever I'm switching flours, I do it all at once rather than changing it out gradually. Sometimes there's a drop in leavening power or a negative change in aroma or texture, but these are temporary until the microorganisms sort themselves out in the new norm. It may transition quickly, or it could take up to two weeks. Plan to feed at least twice per day, or park it in the fridge when you can't. Adequate feeding keeps things moving in the right direction. Just adjust your feed rate according to how fast it's ripening.
Let me know if you have any problems, as I'm sure I've seen it before. :)
Best,
dw
I am having some troubles. At least, I think I am. Let me back up and share how I got here.
I had a beautifully healthy rye starter. It was vigorous. It would grow 3.5 times in volume in 10 hours. (I was once told that rye starters don't grow as much as white starters on account of the lack of gluten...makes sense.) But mine grew and was super airy in the middle. The problem was, it couldn't raise bread. My loaves were flat. Sour and tasty, but extremely flat. My conclusion was that for whatever reason, it was accustomed to getting rye for food and wouldn't raise anything not made of pure rye. So, I decided to go the opposite route. I decided I would train my starter for lift. After achieving this, if I wanted to pull back and put some sourness back in, I would do so. But first thing's first...lift, lift, lift.
After reading your posts, I determined that meant I should change to a white/stiff starter. I decided to make the shift to white first. I'd been feeding my starter at peak 1:2:2. (60% KA AP, 20% organic rye, 20% WW. I want to be able to make any kind of bread with this starter, so my thought was to use different kinds of flours to keep it from getting picky like my last starter. I don't know if this is valid, but it's what I did). Things were progressing slowly. After several days and right around the time of our last conversation, I changed to 1:1:1 (keeping same flour ratios) in the hopes of speeding things up. Of course, this meant feeding more often. Ambient temperature of the house is 70 degrees F and the starter peaks in about 12 hours. The problem? It's barely doubling.
I had been keeping 50g of starter, so I'm doing 50g of water and 50g of flour, naturally. I was wondering if I was just getting a bad read on the height with so little (although I didn't think so). Last night I did 100g:100g:100g and it didn't even double. It only rose 1.5x. The other thing is that it takes a long time to fall. I wanted to make sure I wasn't misreading the peak, so I left it one time a while to make sure it really was peaked. It was. It takes probably 3 hours to start falling in any obvious way.
I've been at this almost a week now. Am I just not being patient enough? I have a hard time thinking so because I'm not even seeing small improvements.
Starter does pass the "float test" but it's not as airy as my old rye starter and I'm under the impression it should be more like tripling in 12 hours.
(Not a thiol victim. No weird odors and starter is always glutenous.)
Any advice is appreciated. Thanks!
From what you describe, it sounds to me like there was nothing wrong with your starter. My sense of the situation is that you are relatively new to sourdough baking, am I right? I don't know what your experience level is with bread in general, but sourdough is different and more challenging. Whole grain sourdough is another level beyond that. Clearly you've done a lot of reading, but unfortunately reading can't substitute for skill, and that is won through practice, observation, and experience.
Not mixing to proper development, fermenting too little, or too much, not dividing at the right time, not shaping properly --- these and more are all things that can lead to flat loaves, and your culprit is far more likely to be one or a combination of these than a starter unable to handle wheat. Is there a class you can sign up for in your area? Having a teacher to guide you is the quickest way to learn how a proper dough looks and feels at every stage.
For starter maintenance, I recommend reading and following Maurizio's guidelines at The Perfect Loaf until you have produced some successful loaves. Something basic made with white flour is the best place for beginners to start, and practice the same loaf until you can do it consistently. Keep at it and you will get it. :)
https://www.theperfectloaf.com/sourdough-starter-maintenance-routine/#more-1429
Best Baking,
dw
It would be great if, in the process of responding to BB's questions, some attention to the RANGE of proportions in feeding (and other variables, like time and temp) might get some additional attention...at least for those of us with potentially more freakish starters.
E.g., I feed my 100% hydration starter 2x/day, and each time the ratio of starter:flour:water right around 1:10:10 (Baker's Math: 100% Flour, 100% Water, 10% Starter), using cold tap water and fermenting in a 68F kitchen. During the summer the amount of starter drops even more (5-8%). My levain is built on the same proportions, and my formulas generally have 15-20% of the total flour in the levain. My dough ferments in 3-4 hours (but at warmer water and room temps: ~78F), and they are fairly, but not excessively mild in acidity.
So I can't imagine using 50-100% of the flour weight in starter in a feeding or levain build (but maybe I should try).
Again...I'm mostly adding this to highlight the range of what might work (and to get some feedback on how much of an outlier these numbers are)...
I don't specify ranges because the variables affect the boundaries. No matter what I say, there will be conditions in which they wouldn't hold up. They're moving targets. I talk about relationships rather than absolute values most of the time, because everything is conditional or relative to something else in sourdough. Flour, hydration, temperature, time, number of propagation steps, the species, their condition and current balance between the populations, along with your goals (intention is an important part of the conversation), all inform your proportions in feeding ...
... because it's never that simple with living things. :)
Beautifully said!
Thanks Debra for you comment. It is an affirmation of the journey I have been on for the past year, learning to pay less attention to my stopwatch and dial in my senses.
I also appreciate the basic idea your last comment expresses...but at the same time, I think there are some productive areas of inquiry to be discussed further, and questions for baker's regarding the limits and trends in feeding and what they can tell us about our own experience and realizing our intentions.
Part of what drove my last post was not only the common references to 1:1:1 or 1:2:2 feeding ratios, on the one hand, but also comments by famous bakers and bread book authors who talk about asking our poor little beasties to take on too big a meal in one sitting (so I'd like to better understand the scientific explanation for the latter comments in particular). Or in other words...what happens in terms of metabolism and population dynamics as feedings get bigger and bigger (besides what we have established, like reduction in acidity), and what implications do those effects have for baking. I imagine that practically speaking, we eventually see longer times to reach a peak, and less acidity, but I wonder if there are any worthwhile points to understand in terms of what is going on with yeast and LAB's as we keep them extra well fed and their environment fresh (?)...
thanks again...
It's a very big discussion --- a book's-worth, really. And unfortunately there's a limit to my time, so I'll not try to cover all the factors contributing to population dynamics here and now. Part of the appeal of the 1:1:1 or 1:2:2 ratios so common here is simplicity, especially for newbies and non-professionals who often equate higher feed rates to more complicated math or wasting flour. The low rate may or may not be optimal for a given starter, depending on the temperature, hydration, ripening time and other considerations. It's never just one thing. But I would like to point out that there is a difference between small batch fermentation in the average home kitchen, and large batch in a production bakery, which is where many famous bakers and bread book authors are coming from and where they develop their formulas.
There's a phenomenon that has been dubbed the mass effect. Somewhat vague, in that there doesn't seem to be any clear or detailed definition/description/explanation of it. I haven't found one, anyway. Large masses of dough or starter tend to move quicker than small --- that is easy enough to observe, and to attribute at least partly to greater heat retention. But there are other claims large-batch bakers make as well. About superior flavor and quality of the finished bread that are probably related indirectly to mass, but not directly to temperature.
Another factor to think about is something I like to call the 'bakery effect' (after the winery effect in wine fermentations). Basically, the bakery setting is contaminated by a buildup of the dominant sourdough microorganisms, due to the volume and frequency of bread-making there, that a bakery itself contributes to inoculation of refreshed starter and new batches of dough. This carry-over is acknowledged in the scientific literature, and explains why all the starters kept in a given bakery tend to return the same organisms (and often some bakers' yeast if that is also used in the bakery), regardless of the type/source of flour -- white, whole wheat, or rye -- and other process parameters. Bakers' yeast will invigorate things, as does continuous propagation.
So there are a lot of subtle reasons for production bakers to be using different feed rates than the average home baker (including bakery hours and work flow). Differences that neither may be aware of if they haven't experienced both. Then of course there are individual differences between starters in general. The scientific literature also says that sourdough processes should be optimized on a starter by starter basis. Keep these things in mind as you're comparing your processes to someone else's, otherwise it can lead to either doubting yours, or dismissing theirs. How much and how often do you bake bread? That will influence whether your home kitchen and bread-making equipment is more like mine, more like a bakery, or somewhere in between.
Having said all that, 10% starter with 100% hydration doesn't seem atypical to me. Note that 10% represents just one more generation than 20%. I'm a home baker who doesn't bake bread very often (my batch sizes are also smaller than most at 1K or less), and I generally use 20% starter for a 12-hour ripening period, to 50% for about 8 hours, 100% for 6 hours or 200% for 4. Variable, of course, with the temperature in the kitchen. I know of one world-class baker who claims to inoculate with as little as .5%. Now, I couldn't get away with that, but then, my kitchen isn't spurring it along.
All else being equal (always the qualifier, seldom the case), reducing the inoculation in starter will increase the ratio of LAB:yeast if ripening time is adjusted to match. But because high LAB suppress yeast, there will come a point at which leavening will drop off. For me that is around 10%. For you, something lower. In a busy bakery, ...? For dough it's often the opposite. Dough is less fermented than starter and has salt added (and lower hydration than 100%, usually). The combination changes the dynamic. But today, I shall leave it at that.
All best
Hi Debra
At last - a potential source of the information I have been scouring the net for, and up to this point failing.
I am fructose intolerant, which to this point has seen me eliminate bread from my diet, as although many varieties will confirm they contain 'no fructose'....as in "why would we put fructose in it?"....it still contains sucrose (either remaining after fermentation or even added afterwards, and i can't quite get them to understand that sucrose is 50% fructose :)
When you have this sort of response, you lose all faith in the integrity of any information supplied on the subject by manufacturers....a bit like with gluten, when a manufacturer claims that it is 'gluten free'....but they suggest you don't eat it if you are actually gluten intolerant :)
Anyway, i came across Sourdough, and I have been wondering whether this might be the 'miracle' I have been looking for - a way to eat bread (yummy), because the fructose in natural sucrose of the flour has been consumed by the starter.
From reading various texts, including yours, it is clear that not all fermentation will consume fructose, and so i am hoping you might shed some light on how i might optimise Sourdough baking to optimise the removal (metabolism) of fructose, so that I can add another food stuff to my limited diet. I imagine that the choice of starter is essential in this regard, as well as the choice of flour (rye being higher in sucrose than wheat, for example).
My finger are crossed that this isn't just blind optimism - can i really add something else to my food list?
Thanks
Urica
Hi Urica,
I'm afraid I can't help you, as I'm not familiar with your condition or its etiology, and I'm not qualified to give diet advice to people with health problems. If you are self-diagnosed, I suggest seeking guidance from a healthcare professional and/or a licensed dietitian.
Best of luck,
dw
Hi DW
I completely understand.
Let me re-frame that question, so that all the risk is on me, and I am not asking if I can eat sourdough, or whether you would recommend that I eat sourdough.
"How does the fermentation process impact the fructose content of the dough?"
Thanks
Urica
The majority of free fructose is likely used up by LAB. But there is also fructose locked in sucrose and some of the more complex carbohydrates of flour. If you don't tolerate sucrose, then the fructo-oligosaccharides are likely to be a problem too. It's not very likely that they would be entirely broken down.
My best,
dw
I must confess, my degree is in business and accounting. I've been working with sourdough for some time now and have grown to love the nutritional benefits. I'm trying to read and understand the thread, and of course I understand LAB, different sugars, the process that breaks down the complex sugars in wheat, to simple sugars and then are consumed by the bacteria and yeast. (I know this is an over simplification). My goal is to understand what improves the consumption of sugars (i.e., whole grain, white flour, etc...) and the amount of time, all other things being equal, does it take to do so. My grandchildren do not yet like a very acidic (sour) bread. My goal is to ferment long enough and to utilize the best combination of grains (whole or flour) for the bacteria and yeasts to consume the bulk of the sugars, yet not so long as to make it very sour (more mellow).
I appreciate over simplification if possible.
Marcy
PS are you still teaching courses?
I'm taking a break from teaching this year, but I'll be back at it in 2020 :) Thank you for your interest!
For milder breads I'd recommend maintaining your mother starter on white flour at a low hydration (firm dough), and feed it 2 or 3 times per day at a suitable rate, at room temperature (for at least the day or even two leading up to making your dough if you can't keep that up indefinitely -- I get it). Whatever it needs to invigorate the leavening and reduce the souring, so play it by ear. Aim for a starter that can expand to triple in volume, and not deflate too much by feeding time. Adjust the feed rate as needed for the schedule.
Also, pick a formula and process that is meant to produce mild bread, like Jeffrey Hamelman's Pain au Levain. When fermented properly and well-made, I find that one to be popular with children (and adults). But you don't really want all the free sugars to be consumed because they are important for browning and flavor. Without them, your loaves will be pale, unattractive, and not as flavorful as they could or should be.
My best,
dw
For the last fortnight I have searched for and read / reread Debra Wink's posts on the Fresh Loaf website. There is a lot to learn and unlearn about sourdough. I am grateful to you both for maintaining the site and helping me with the art and science of sourdough.
I was wondering if there is a list of known co-substrates that can be used to facilitate formation of acetic acid (mainly in the interest of reducing acetic acid production in my own stiff starter, or in experimental doughs it would be used in.)
It seems to be pretty confusing since I'm really rusty on chemistry, but I remember something about reducing sugars...which are reducing agents, but then you've got the electron acceptors (oxidizing agents) which you mention to be co-substrates.
Also, without a co-substrate, would maltose be fermented into lactic acid? (Or I guess the question is what other acids are potential byproducts of fermented maltose. Because I don't think yeast in sourdough can use maltose directly, correct?)
And another thing comes to mind -- what about lactose? Milk is often an interesting addition to doughs, but I was curious about how it would be fermented (I'm pretty sure the yeast in sourdough can't use lactose as is).
I was wondering if there is a list of known co-substrates that can be used to facilitate formation of acetic acid (mainly in the interest of reducing acetic acid production in my own stiff starter, or in experimental doughs it would be used in.)
I'm not aware of one. That was just a list I compiled as I came across them in various articles, but most of those things are not present in flour in significant amounts. Fructose is probably the most important (along with pentose sugars in ryes and high-extraction flours). So, if you want to keep acetic acid to a minimum, I recommend sticking to the refined white flours in your starter and limiting whole grains, if using, to your final doughs. Beyond that, keep the starter cooler if you want to reduce total acid production to make it milder overall, or keep it warmer if you want to increase lactic acid and total acidity.
Also, without a co-substrate, would maltose be fermented into lactic acid?
Yes, either all lactic (homofermentation) by the facultative heterofermenters, or half lactic/half ethanol by the obligate heterofermenters.
I don't think yeast in sourdough can use maltose directly, correct?)
Some yeasts can, but they generally don't while other sugars are present in high enough amounts, particularly glucose.
And another thing comes to mind -- what about lactose? Milk is often an interesting addition to doughs, but I was curious about how it would be fermented (I'm pretty sure the yeast in sourdough can't use lactose as is).
Baker's yeast doesn't utilize lactose, but I'm not sure if that's true of all sourdough yeasts. Certainly, there are several lactobacilli that can ferment lactose as that is how yogurt is made. And milk adds to bread in other ways.
My best,
dw
Hi Debra. I truly admire your sense of sharing and educating when it comes to bread making. I have a question or two regarding my starter. Well, I am pretty happy with it. It's vigorous and mild. It's just after I feed it regularly with atta whole wheat (50% hydration), it has pleasant alcoholic smell and mild sweet taste (literally sweet) with a hint of acetic smell when ripe. When nearly ripe, it smells like donut shop (lol). Do you have any thought about it? I never measure temperature, but it gets somewhere between 26 to 38 degree celcius where I live. I ferment at room temperature for 2 hours before refrigerating. I'm not trying to change a thing, just trying to get better understanding of it.
Another question. Could you name any literature regarding what sugars contained in every type of grains?
Thanks, I appreciate it
Jay
... it has pleasant alcoholic smell and mild sweet taste (literally sweet) with a hint of acetic smell when ripe. When nearly ripe, it smells like donut shop (lol). Do you have any thought about it?
I'm thinking it sounds pretty wonderful :) I would say that you have found what works best for you with your atta flour in your warm climate. Whole wheat and high temperatures tend to encourage bacteria and the sourness/havoc they bring when out of control, but your low hydration and judicious use of the refrigerator are the counter measures keeping all that in check.
And that hint of acetic is quite typical with low hydration and whole grain, but acetic acid is higher only in relation to lactic acid and not more acid overall (acid balance rather than acid quantity). Acetic acid can be higher in relation to lactic acid and still be low in concentration (mild in flavor). You're keeping the total acid concentration in check by keeping bacteria in check.
Could you name any literature regarding what sugars contained in every type of grains?
I'm sorry, I haven't come across anything comprehensive in that way.
My best,
dw
I appreciate it. And regarding my second question, I just wondered if using non-wheat flours entirely in levain for mostly wheat bread can always be done seamlessly. I have tried using 66% oat flour in levain. The final rise was fast, although vegetal taste was present (almost like cucumber lol)
Right now I have levain made with entirely glutinous black rice flour (made with my atta starter) in the fridge. If it works, I will convert my starter to rice starter. My rationale is, starter has no structural role anyway, and since I like black rice flour for nice crust color (even at 5%), maybe worth the shot.
Best regards,
Jay
As far as prefermenting various non-wheat flours in your levain (as opposed to adding them to the final dough?), you just have to try it on a flour-by-flour and bread-by-bread basis. I think that fermentation generally either magnifies an ingredient's flavor or transforms it into something different. And the particular species and strains of bacteria and yeast in your starter play a part in the resulting flavors, not just the substrate. Interesting that you got a cucumbery flavor from oat flour. I wonder if scalding or cooking the oat flour first would give you yet another flavor.
If it works, I will convert my starter to rice starter.
Just keep in mind that maintaining your starter on a different grain is likely to change the microbial profile within, and that can change its character. Only you can decide if the change is desirable or not in the end. To be safe, keep a portion of your starter the way you have been (or stored in the fridge) while converting the other portion. It may take a few weeks to transition and get a good idea about how it is going to turn out in the long run.
Best in baking :)
dw
The black rice levain was vigorous. I usually proof for 4 hours, but tonight's bake was overproofed at 3.5 h
Regarding putting weak flour in my levain instead of final dough, my intention is to preserve the gluten of my strongest flour, so I can fit in more whole grains without compromising structure too much, by letting it spending less time fermenting. For example, tonight's bake, I managed to fit 29% black rice flour in 75% hydration dough. The dough could be grabbed by hands. All whole grain went to levain. Since all gluten was in my white flour, no need to worry about gluten degradation in the levain. Unfortunately, since it was overproofed, I can't say much right now. But I am determined to perfect it, since I really want 30% black rice in my daily bread rotation :D
Regarding oat flour, I tried regular rolled oat vs. instant oat vs. toasted rolled oat. Instant oats give the strongest vegetal flavor, toasted oats give more acidic flavor, and regular rolled oats give mild bread that's highly versatile. Rolled oats one is in my daily bread rotation. 20% rolled oats + 20% atta, to be precise
And regarding my starter, she already has changed complexion from light brown to deep purple :D
Regards,
Jay